Chloe Clinical
All-in-One Decentralized Research Platform
Unlimited Trials,
Limitless Possibilities
Chloe Clinical provides unlimited access to fully functional, decentralized clinical research, from prospective observational studies to randomized, placebo-controlled trials. With the ability to collect robust health data right from a participant’s home, you can launch and manage multiple studies without traditional constraints.
Unlimited Research
1-10K+ Trial Sizes
IRB Approved
90%+ Completion
Partners we work with
We believe budget should never be a barrier to rigorous, participant-centered research.
Chloe is flexible and customizable to fit a variety of needs.
Our Platform
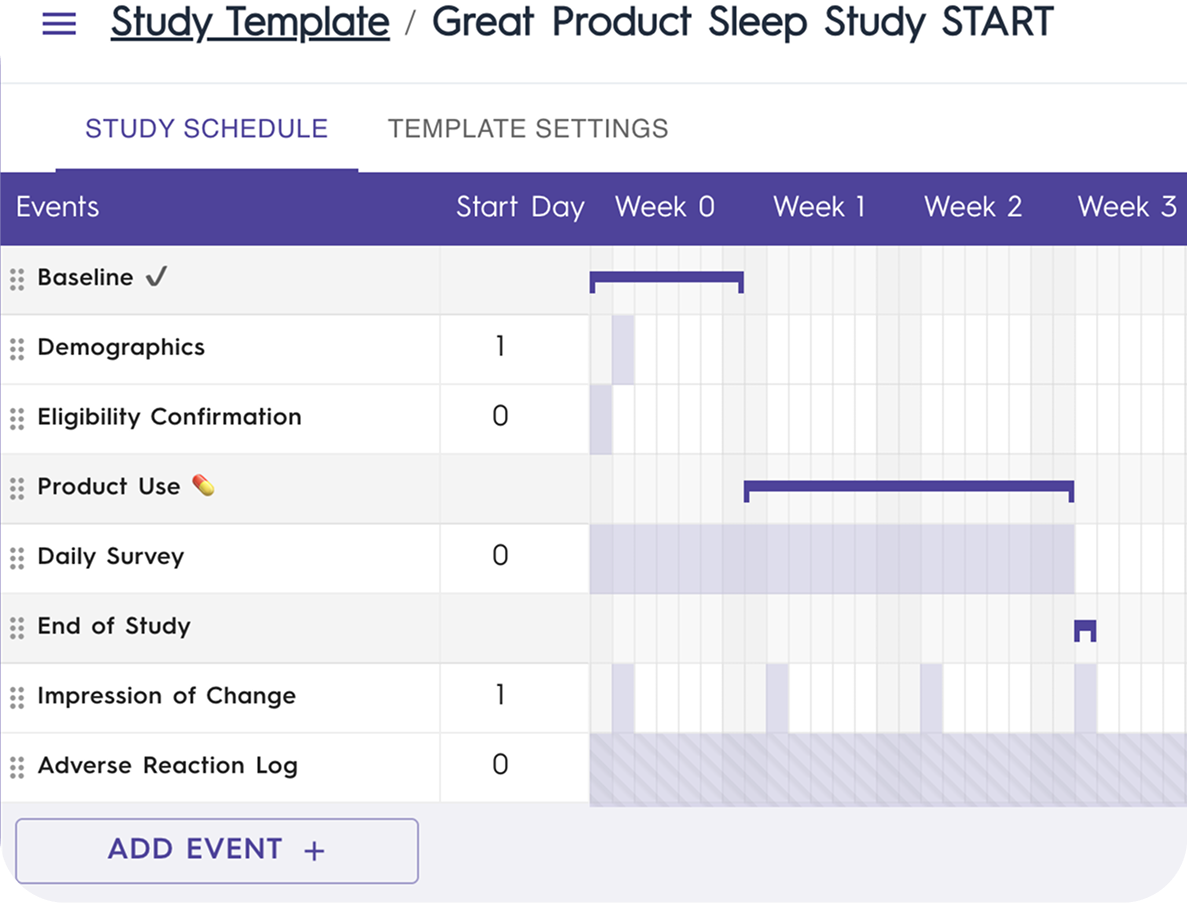
Design Your Study with Ease
Flexible, Modular Study Setup: Choose from a ready-to-use library of protocols, tasks, surveys, and trial tools, or customize your own.
Automated Engagement & Retention: Streamline participant management with scheduled events, automated reminders, and personalized messages to keep participants on track.
Simplified Participant Randomization: Effortlessly assign participants to study arms with built-in randomization tools.
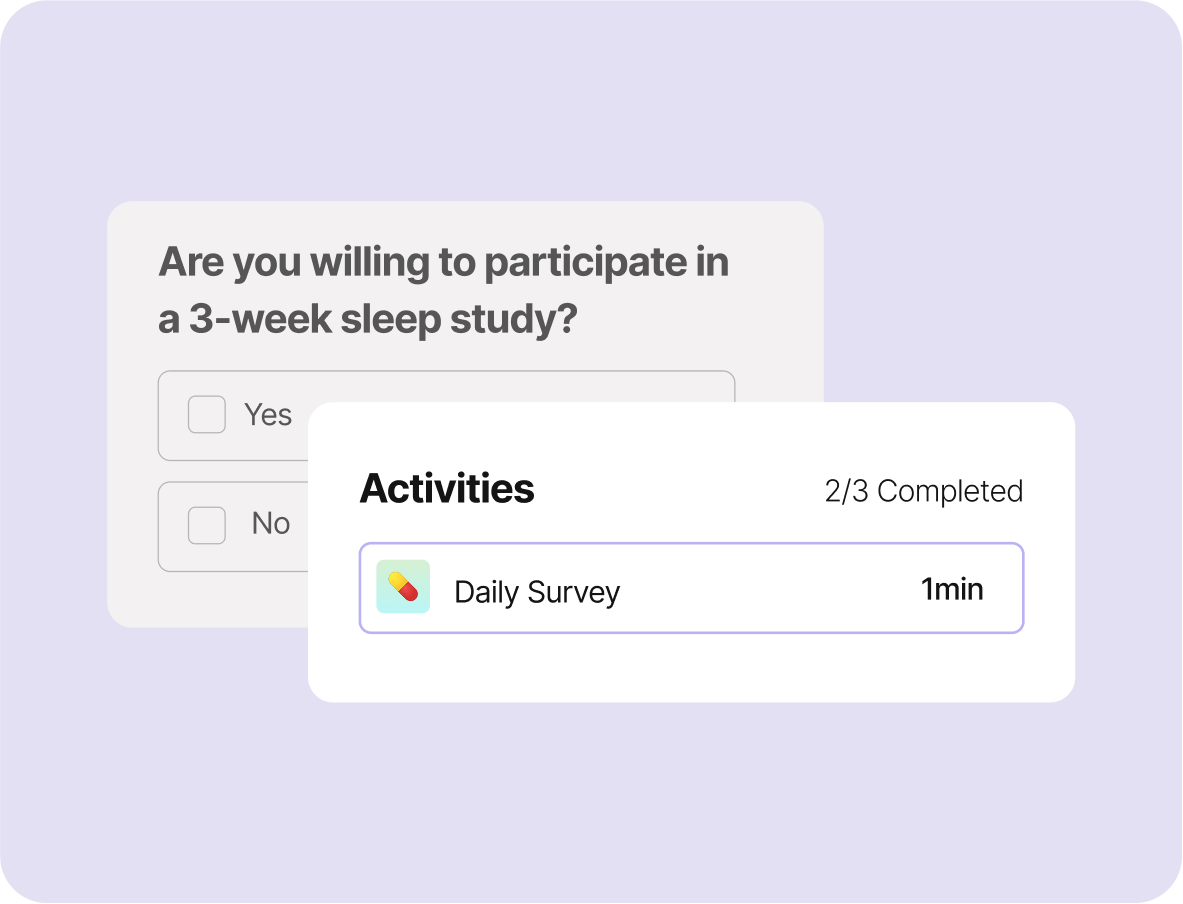
Control Recruitment & Optimize Participants’ Experience
Scalable, Inclusive At-Home Enrollment: Expand your study reach with efficient digital recruitment. *A recent People Science RCT recruited 150 participants in 11 days.
Streamlined Digital Consent & Onboarding: Easily invite participants via personalized text or email links, with integrated eConsent and automated eligibility screening.
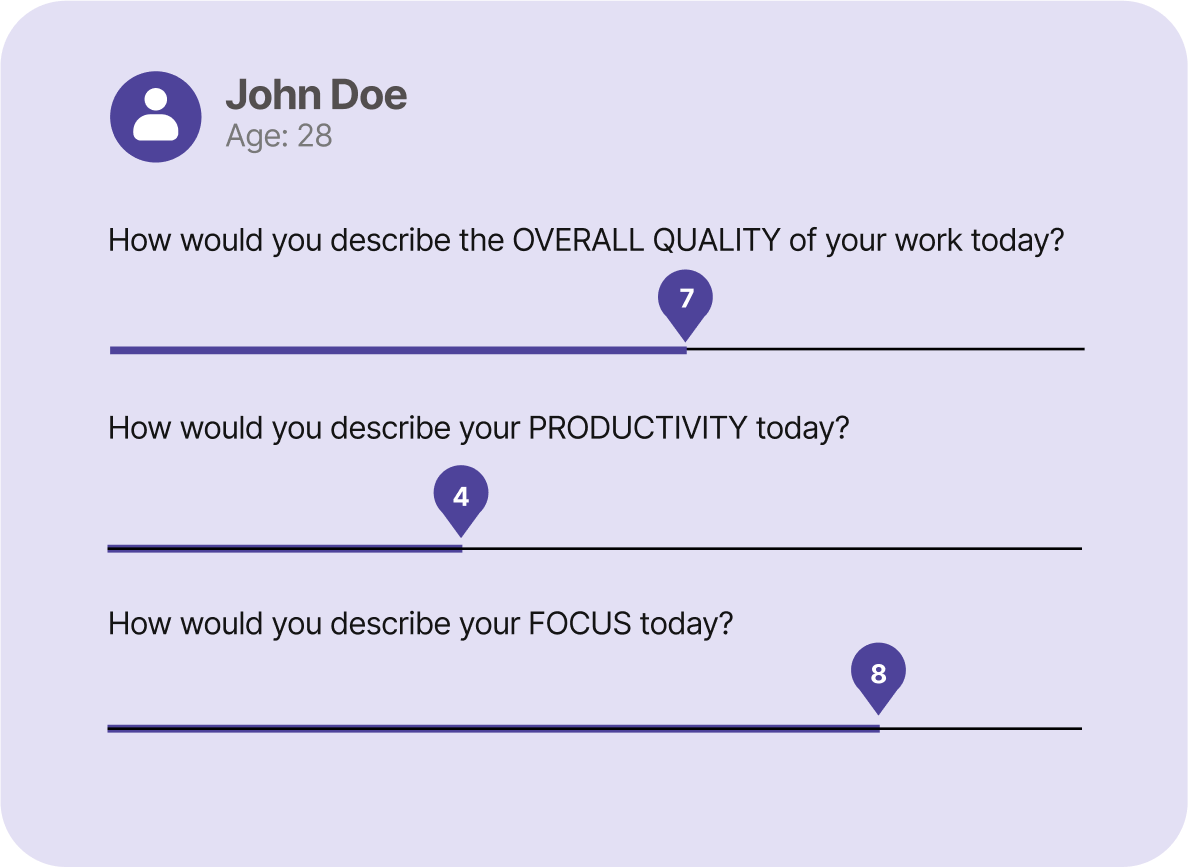
Participant-Centered Design: Simplify the experience with at-home data collection and protocols, up to 3x faster and driving 90%+ compliance and retention.
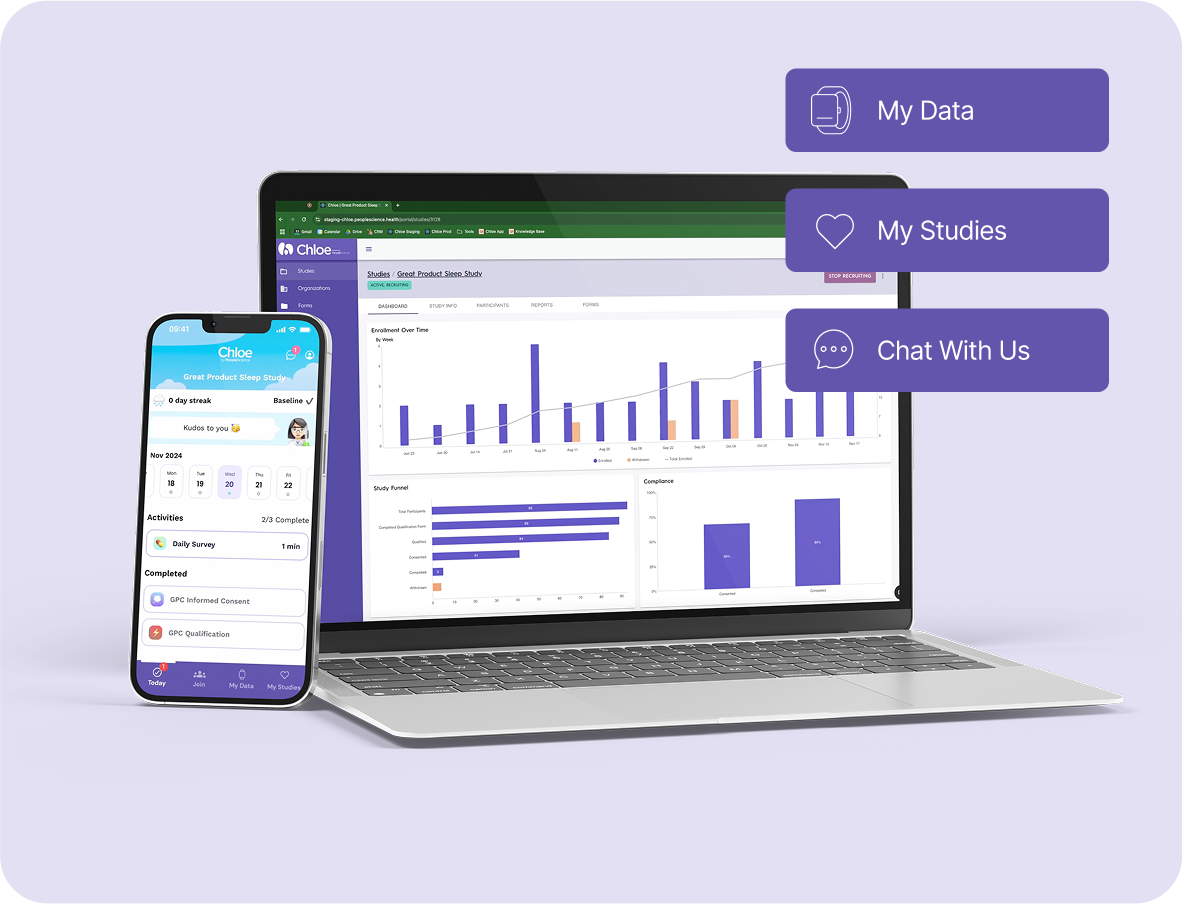
Real-time Monitoring and Adaptive Study Management
Always-On Access: Available on both mobile and web, Chloe Clinical fits seamlessly into any schedule or workflow.
Continuous, Real-World Data: Capture both passive and active data in real time through smart device integrations for objective, up-to-the-minute insights.
Adaptive Protocols: Completely customizable workflows improve outcomes and minimize errors.
Instant Participant Feedback: Communicate directly within the app for timely feedback and stronger engagement.
Custom Data Insights and Reporting
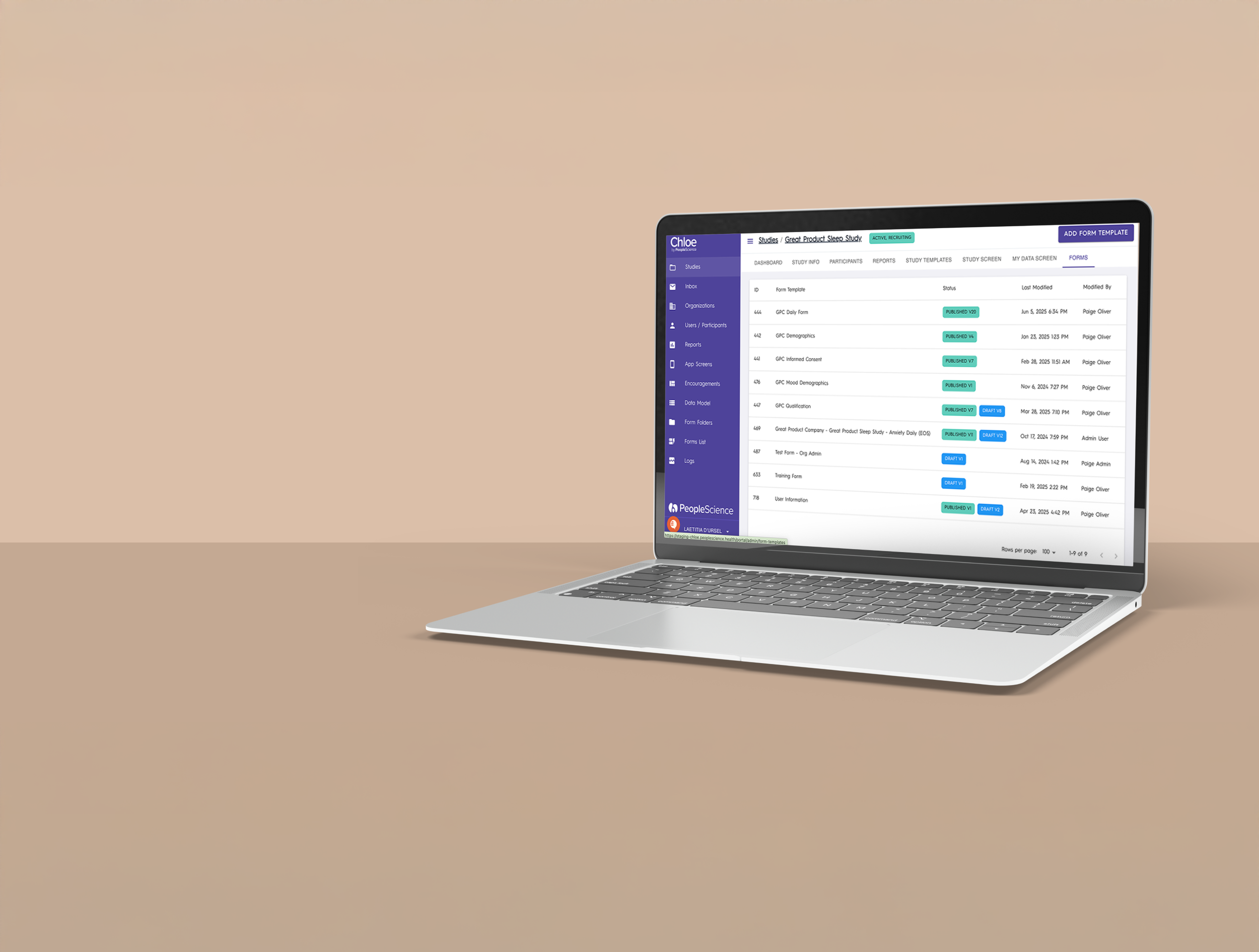
Dynamic Dashboards: Instantly visualize key study metrics like compliance, retention, and engagement, through real-time dashboards.
Custom Reports: Generate tailored reports to drive decision making among key stakeholders.
Participant Transparency: Option to easily share study results with participants via in-app visualizations and statistics to build trust and reinforce engagement.
Seamless Export: Export data and reports for analysis, submission, or internal review.
What you can study with Chloe Clinical
Chloe supports a wide range of study areas, product types, and study types, as well as both subjective and objective participant inputs.
-
Study how interventions impact a wide range of health areas:
Women’s Health
Metabolic Health
Human Performance
Microbiome
Longevity and Aging
Cognition and Mood
Sleep
Dermatology
And More!
-
We support rigorous, real-world studies across a wide range of interventions:
Dietary Supplements (vitamins, minerals, amino acids)
Functional Foods and Beverages (fortified cereals, dairy alternatives)
Herbal and Botanical Extracts (turmeric, ginseng, ashwagandha)
Probiotics and Prebiotics (gut microbiome support)
Omega-3 Fatty Acids (cardiovascular, joint, and cognitive health)
Protein and Antioxidants
Medical Foods
Wearable Devices
Home Test Kits
Digital Health Interventions
Cannabinoids & Psychedelics
Family & Pet Health
Cosmetics and Personal Care Products
-
To develop products that meet needs and deliver meaningful outcomes, it's important to build a thoughtful study roadmap. Chloe Clinical supports this journey with flexible tools that grow with you:
Exploratory and pilot studies for early signal-detection.
Randomized, double-blind, placebo-controlled trials (RCTs) for gold-standard validation.
Consumer perception studies to capture real-world experiences.
Longitudinal studies to track outcomes over time and understand sustained impact.
Participatory research that puts individuals at the center.
N=1 designs for patients and self-trackers.
-
Our privacy-first platform collects diverse, high-quality, objective and subjective data:
Wearables: Real-time data like heart rate variability, sleep, temperature, activity, and glucose from devices like Apple Watch, Fitbit, Oura Ring, and Dexcom CGM.
Biomarker Sampling: Blood, saliva, urine, stool, hair, and vaginal swabs collected via accredited labs and home kits.
Validated Questionnaires: 60+ IRB-approved surveys capturing behavior and psychology.
Custom Surveys: Measure lifestyle and social factors for deeper participant insights.
And More!
See how leading brands are benefitting from Chloe Clinical.
PEER REVIEWED PUBLICATION
Probiotic Anxiety Study on the Gut-Brain Axis
DOUBLE-BLIND, PLACEBO-CONTROLLED, RANDOMIZED, DECENTRALIZED TRIAL
THCV Improves Energy and Wellbeing with Minimal Side Effects
IRB APPROVED RCT
Exploring Effects of a Novel Infant Synbiotic
Journals Featuring Our Team’s Research
Frequently Asked Questions
-
Decentralized trials are conducted remotely, right from participants' homes, using tools like the Chloe app for data collection, communication, and engagement. This model, originally pioneered by our co-founders in the pharmaceutical space, makes studies more inclusive, efficient, and scalable. With Chloe, you can reach more diverse populations, reduce logistical burdens, and accelerate timelines without compromising on scientific rigor.
-
Absolutely. Chloe is built for decentralized, hybrid, and traditional trial models, enabling flexible study designs and participant access.
-
Chloe currently supports studies across 9 countries, including the United States, Australia, Canada, Germany, Netherlands, New Zealand, Norway, Spain, and the United Kingdom, making it easy to run multi-site, cross-border research under a single contract. And we’re adding more as we speak!
-
Our platform runs decentralized clinical trials, which makes it easy to design and launch studies quickly, often within just one month.
Recruitment is faster too; for example, we recruited 150 participants for a sleep study in under two weeks. Automated processes streamline many tasks, making study management more efficient.
Plus, you can access real-time data during the study to monitor recruitment, compliance, and completion, and download data at any time during and after the study closes.
When the study ends, our AI-powered and biostatistician-reviewed analysis services speed up data review, helping you gain insights faster.
-
Chloe was built to make clinical research accessible. For emerging brands or startups without in-house clinical teams, our platform can be paired with full CRO services. We’ll handle everything from protocol design to execution, while you stay in the loop through real-time dashboards, with no need to chase down updates. You stay in control, with your studies centralized in one place and always at your fingertips.
-
For experienced teams, Chloe offers deep customization and full alignment with pharma-grade best practices. It’s designed to enhance your existing workflows with efficient, decentralized and hybrid-ready tools, making trials faster to launch, easier to manage, and more cost-effective. Whether you're running one study or a portfolio, Chloe helps streamline execution while maintaining scientific rigor.
-
Yes. Studies run on Chloe meet IRB standards and follow regulatory-grade protocols, making them eligible for peer-reviewed publication. Several Chloe-powered studies have already been published. And if you’d like support, we offer services to help write and submit manuscripts, making the path from data to published insight easier than ever.
Ready to Research?
Streamline data collection to drive faster, more insightful outcomes for your brand. Experience Chloe Clinical firsthand.