CASE STUDY
Decentralized Excellence:
People Science and Verb Biotics Publish Randomized Control Trial of GABA Probiotic Lp815 for Symptoms of Anxiety in Beneficial Microbes
Study Overview
A look at the study facts, key findings, and real-world implications.
Study Snapshot
Primary focus
Clinically evaluate the impact of two doses of LP815 GABA probiotic versus a placebo on symptoms of anxiety. Drive product development, support claims and generate peer-reviewed published research.
Participants
83 participants, 63% female, 64% Caucasian.
Study Type
Double-blind, placebo-controlled, randomized, decentralized trial approved by the Advarra Institutional Review Board (IRB).
Study Structure
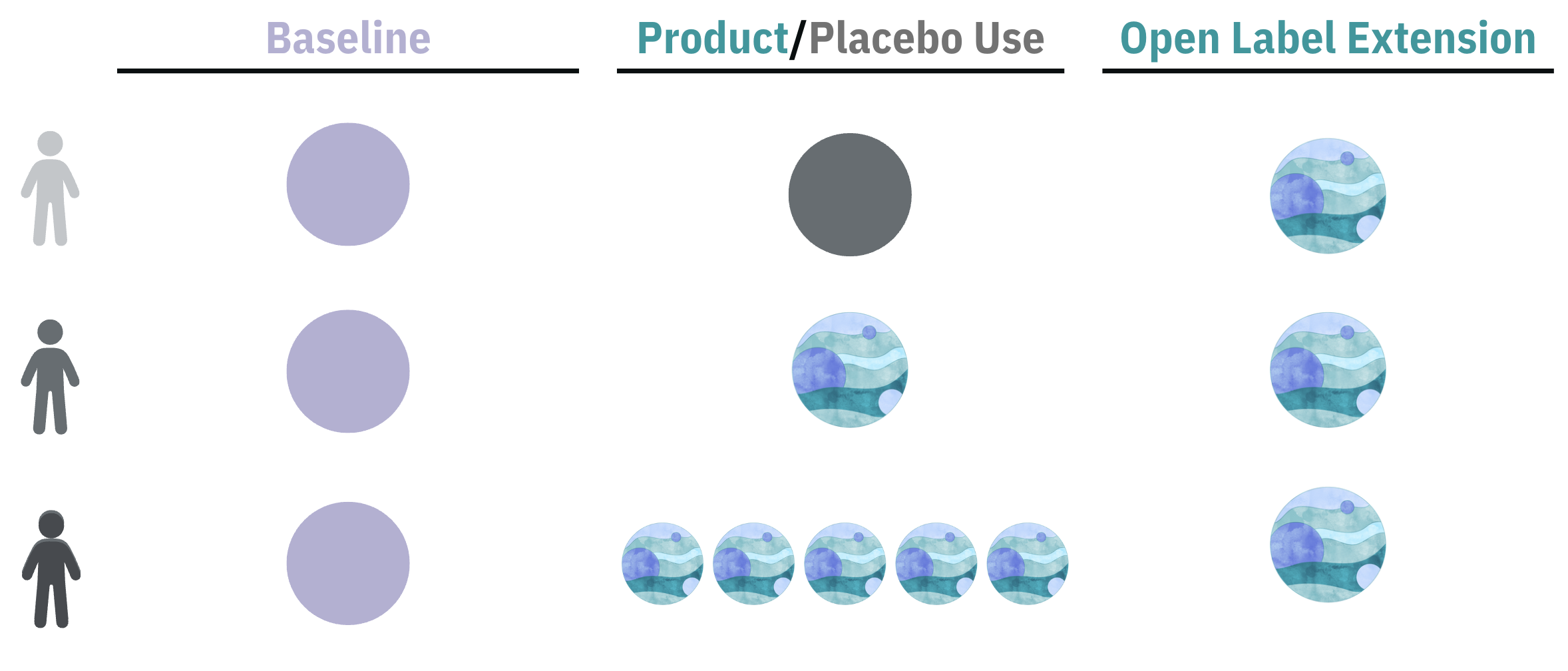
Participants collected one week of baseline data followed by 6 weeks of product or placebo use. Participants could opt into an Open Label Extension. This study was completely decentralized. Outcomes were collected in Chloe mobile app.
Duration
3 weeks recruitment, 1 week baseline, 6 weeks intervention.
Analytical Methods
Non-parametric statistics (Holm’s and Dunn’s corrected Kruskal-Wallis) and Mann-Kendall trend over time tests.
Validation
IRB approved. Peer-reviewed publication in Beneficial Microbes.
Key Results
Publication
Peer-reviewed publication in Beneficial Microbes
151%
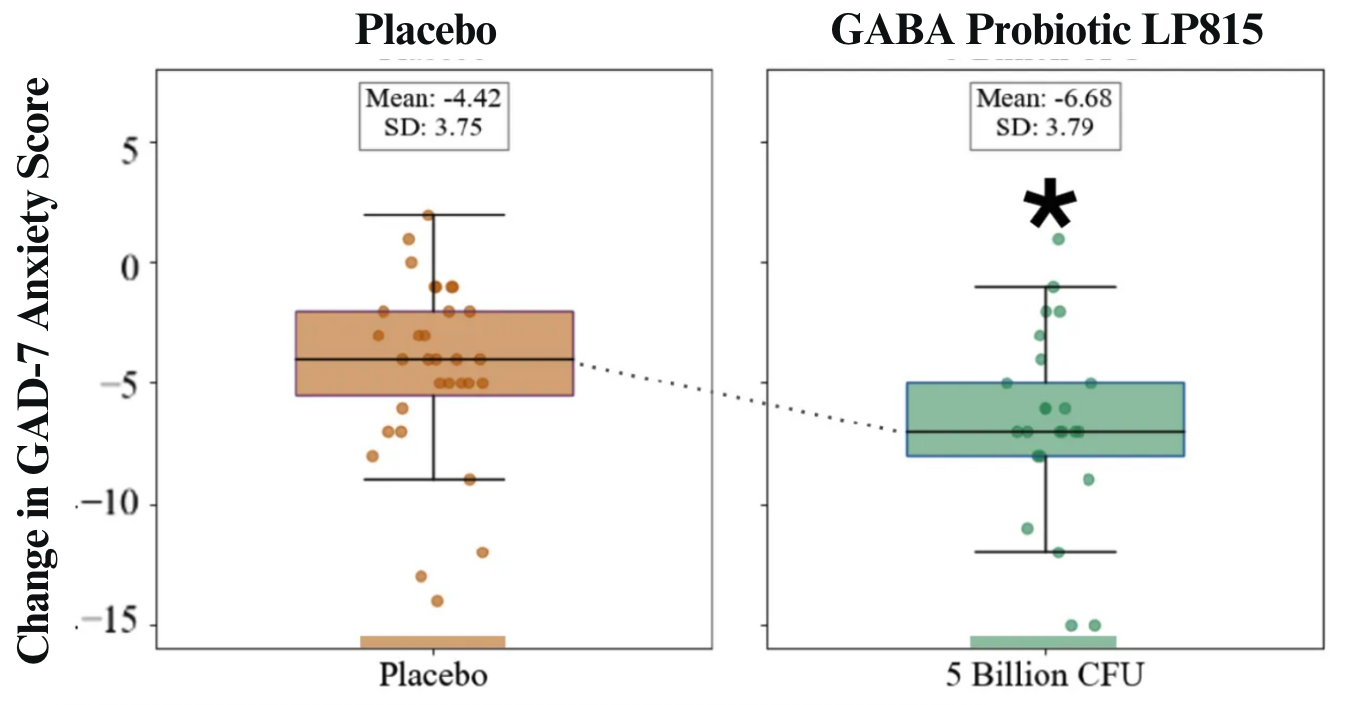
Statistically significant improvement in symptoms of anxiety at 4 and 6 weeks. 6.68 vs 4.42 drop in LP815 vs. Placebo GAD-7 score.
p = 0.04
Reduced Feelings of Irritability and Annoyance.
ISI
Trend Toward Reducing Sleep Disturbance Symptoms.
96%
Participants very satisfied, study experience average rating = 9.3/10.
Study Details
Introduction
Verb Biotics, a leader in the development of effective and novel probiotic strains, tested the effectiveness of lactiplantibacillus plantarum 815 (LP815) — a strain selected in the laboratory to produce the inhibitory neurotransmitter GABA in the physiological pH range. Our trial sought to determine if LP815 could impact symptoms of anxiety, sleep disturbance and cognition using the gold-standard research design: a randomized, placebo-controlled trial in a real-world setting.
The major inhibitory neurotransmitter gamma-aminobutyric acid or GABA plays a pivotal role in mood and sleep. GABA exerts sedative and anxiolytic effects both within the central nervous system and through the gut-brain axis, which has generated interest in the potential for gut GABA to modulate mood and sleep. The present study is the largest to date investigating the impacts of a GABA-producing probiotic.
To bolster its marketing claims and differentiate LP815 from conventional probiotics, Verb Biotics partnered with People Science, specialists in technology-enabled decentralized clinical trials. This collaboration gathered concrete scientific evidence for LP815's impact on symptoms of anxiety, poor sleep and cognition.
Data Collection
Participants collected 1 Week (W) of baseline data, using the Chloe app to log their demographics and medical history, anxiety symptoms (validated GAD-7), sleep disturbance symptoms (validated ISI), wearable data and cognition. This was followed by 6 weeks of product or placebo use. The double-blind design ensured that neither the participants nor the researchers knew which group took the active study product, enhancing the scientific integrity of the study. After 6 weeks, all groups had the opportunity to try the 1 Billion CFU probiotic as part of an Open-Label Extension.
Execution
Chloe streamlined study design, recruitment, data collection, monitoring and data visualization, providing a comprehensive approach to decentralized clinical trials.
Recruitment targeted healthy adults with mild-to-moderate symptoms of anxiety. Recruitment leveraged community connections and targeted advertising to ensure a representative population without imposing strict demographic quotas, resulting in a varied participant pool (83 participants, 63% female, 64% Caucasian).
The study spanned 7 weeks, including 1 week of baseline and 6 weeks of daily low dose probiotic (1 Billion CFU), high dose probiotic (5 Billion CFU) or placebo. Intervention adherence rate was 95%.
Study Outcome
The collaboration yielded statistically significant, publishable results. The study found that the GABA Probiotic LP815 reduced feelings of anxiousness, irritability and annoyance and trended toward reducing symptoms of sleep disturbance. Caption: Box and whisker plots of change from baseline in validated GAD-7 anxiety score after 6 weeks of Placebo (left, orange) or 5 Billion CFU daily LP815 TM (right, green). Dots represent individual participants’ data. Those taking LP815 dropped their anxiety by 6.68 points, compared to 4.42 in placebo. That’s 151% greater improvement.
Recruitment completed in just 3 weeks. The project demonstrated People Science’s full-service support capabilities, from study design to data analysis and publication management, leveraging the Chloe platform to simplify every phase of the trial.
“Our trial with People Science was a clear success and allowed us to make the key business decision to pursue both mood and now potentially the sleep market. They offered essential guidance on selection of accurate metrics, executed rapid under-budget recruitment and data analysis and prepared our manuscript within a couple of weeks. The experience and critical thinking of the scientists and staff were invaluable for this trial. We’ll be sticking with PS for our subsequent trial and would highly recommend them to our colleagues.”
Noah Zimmerman, PhD
CSO at Verb Biotics
-
This RCT provided gold-standard evidence that Verb’s GABA Probiotic product improves feelings of anxiousness, irritability and annoyance, strengthening brand reputation and setting the ingredient apart from competitors.
Findings were published in the respected journal Beneficial Microbes, focused on the microbiome and probiotics, adding credibility to Verb’s claims. Peer-reviewed publication enabled Verb to attract media attention and present its findings to the scientific and industry community. Critically, trials like these help validate product claims for Verb’s customers, strengthening trust and confidence in the supplier relationship. This foundation of evidence positions Verb as a preferred partner and “go-to” supplier. Following this trial, Verb expanded its research to explore additional benefits of its products and their mechanistic underpinnings.
-
Enabled seamless remote participation, reducing logistical barriers and costs associated with in-person visits.
-
The Chloe researcher portal provided instant insights into participant data, allowing timely adjustments to maximize study effectiveness.
-
Structured prompts and reminders helped maintain high data quality and participant adherence throughout the trial.
Benefits to Verb Biotics
Benefits to
Trial Participants
-
Allowed participants to track their experiences with the probiotic from home or on-the-go, enhancing accessibility. All participants had the opportunity to try the probiotic at end of study via an Open Label Extension.
-
All participants received personalized in-app results at the end of the study, as well as first access to the overall study results.
-
The platform’s usability scored 4.5/5, with an average rating of trial experience of 9.3/10. 96% of participants were very satisfied, willing to join future studies and likely to recommend Chloe to their peers.
“This app made this whole process super smooth and manageable.”
“I’m loving this study.”
Study Participant Feedback
Other Case Studies

Book a Demo
Turn your customers’ everyday health data into your most powerful competitive asset. Explore our platform Chloe today.