Clinical Research Services
Accelerate your brand with scientific validation
Brands Trust Us.
People Science partners with health, wellness and nutrition brands to turn research goals into real evidence. Whether you need to expand your team’s capacity or have us fully manage your study, People Science experts deliver the insights and validation for your brand’s success.
The Flexibility You Deserve
People Science offers a flexible range of individual and comprehensive services designed to meet the needs of any specific project. With deep experience running end-to-end scientific studies and supporting small partner teams, our services include:
Scientific & Decentralized Clinical Trial Expertise
Design your protocol effectively with expert consultation and study design support.
Gain actionable insights from your data with thorough statistical analysis.
Publish and share your findings confidently with professional manuscript preparation.
Operational Excellence
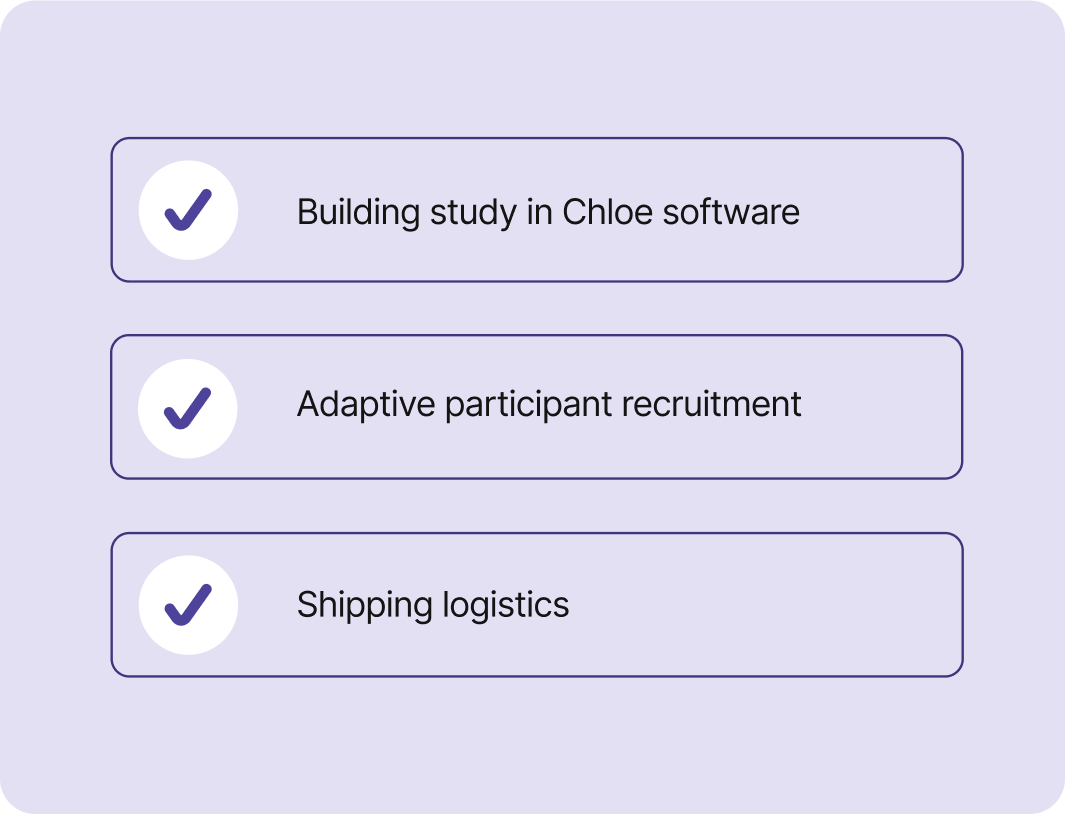
Build your study with a custom Chloe software implementation.
Enroll your target population quickly with adaptive participant recruitment.
Run your workflow efficiently from start up to close out.
Cleanly manage shipping logistics for study materials.
Coordination and Management
Run your study smoothly from start to finish with dedicated study project management.
Ensure participant compliance and retention with proactive participant coordination.
Navigate regulatory requirements confidently with comprehensive IRB/ethics board management.
Streamline lab, test kit, and wearable operations with efficient vendor management.
Flexible Approach to the Science
Generate high-quality evidence with randomized placebo-controlled trials.
Monitor outcomes over time with prospective observational studies.
Measure change effectively with baseline-controlled, longitudinal studies.
Evaluate real-world impact with consumer insights and clinical outcomes from commercial products.
Track patient progress in real time with clinical monitoring in healthcare settings.
Select the best services for your needs and budget with People Science’s flexible, adaptable research solutions.
Why use People
Science Research Services
Our team is composed of veteran research scientists, medical doctors, clinical managers, and coordinators with decades of experience across academic research, the pharmaceutical industry, and in running decentralized clinical trials. This unique blend of expertise enables us to bring rigorous clinical research practices to the world of complimentary and integrative medicine, food-as-medicine, digital health and wellness interventions.
OUR GUIDING PRINCIPLES
Scientific Rigor
People Science studies will always adhere to the highest standards of scientific integrity.
Collaborative Partnership
We work in service of your brand and the science. From protocol design to manuscript development, we strive to understand your needs and achieve your research goals.
Operational Excellence
We believe that efficient and effective processes and workflows unlock better research.
Frequently Asked Questions
-
The Chloe Platform is our self-serve digital research tool for running human health studies quickly and efficiently. Our Clinical Services go further, offering full-service, end-to-end research support for more complex or gold-standard clinical trials, with expert guidance at every step.
-
It depends on your goals. If you're looking to run a quick, real-world evidence study or consumer perception study, Chloe may be all you need. If your research involves clinical endpoints, regulatory oversight, or publication goals, our services can support or fully manage the process. We're happy to help assess your needs.
-
Yes! From refining your research question to protocol development, IRB submission, and recruitment planning, we can guide or manage the entire study design process tailored to your product and goals.
-
As much or as little as you need. We offer a flexible range of services, from one-off support (like protocol writing or data analysis) to fully managed clinical trials, including compliance, coordination, and publication.
-
Our team includes veteran research scientists, physicians, clinical trial managers, and DCT experts with decades of experience in pharma, academia, and wellness research. We blend scientific rigor with a deep understanding of the consumer health space.
-
Timelines vary based on study complexity, but most studies take 4-6 weeks to launch. We'll work closely with your team to develop a timeline that fits your needs and helps you move forward efficiently.
-
Absolutely. We help define your recruitment strategy, create engaging materials, manage leads, and support participants throughout the study to ensure high retention and compliance.
-
Yes. We offer full manuscript development, data visualization, and journal submission support to help share your results with credibility and impact.
-
We deliver rigorous, actionable insights through statistical analysis, complete study reports (CSR), and visual dashboards. We can also tailor data outputs to support claims, publications, or internal strategy.
-
Yes. We’ll work with you to scope the right level of support, whether that’s full-service trial management or just the pieces your team needs help with.
-
We manage all aspects of IRB submission and maintenance, and we’re experienced with ClinicalTrials.gov setup and reporting. We ensure your study is compliant with all applicable regulations.
-
Yes. We handle setup, logistics, and vendor management for lab kits, wearables, and biomarker testing, including partners like Dexcom and Quest. We can also integrate new vendors as needed.

Ready to Research?
Accelerate your brand with scientific validation with our clinical research services today.