CASE STUDY
Empowered Innovation:
People Science’s Chloe Clinical Software Enables Persephone to Explore Effects of a Novel Infant Synbiotic
Study Overview
Introduction
As clinical research evolves beyond the traditional contract research organization (CRO) model, innovative teams are turning to flexible technology platforms that allow them to run studies independently with greater efficiency and control. Persephone Biosciences, a growing company in the biotech space, recently completed an infant synbiotic study named “ARTEMIS” using the Chloe Clinical Software as a Service platform for clinical trial management.
Chloe’s easy accessibility and adaptable form-building capabilities addressed some of Persephone’s primary concerns: reducing participant burden and minimizing dropout risk. This platform allowed them to build a highly complex and advanced clinical trial with the best practices already built into the platform…and they were able to do it all independently. Compared to previous tools, the Persephone team reported that Chloe offered a more intuitive, participant-centered experience.
This case study highlights how Persephone leveraged Chloe to successfully manage the ARTEMIS trial and how the partnership is informing both organizations’ visions for the future of decentralized, technology-enabled research.
Key Outcomes
✔
Significantly Lowered Clinical Trial Expenses
✔
Optimized Persephone Team’s Time
✔
Enabled Persephone to Run ARTEMIS Independently
80%
Adherence Rate
Study Details
ARTEMIS Snapshot
Primary Focus
Evaluate the tolerability and impact of Persephone’s proprietary infant synbiotic (a precision combination of four probiotic strains, prebiotics, and Vitamin D) on the infant and toddler gut microbiome, immune profile, and overall health outcomes.
Participants
136 infants and toddlers aged 2-24 months.
Study Design
Randomized, double-blind, placebo-controlled trial approved by the Advarra Institutional Review Board (IRB).
Study Structure
Subjects received either the synbiotic (30‑day supply) or control. The treatment group received four curated probiotic strains + HMOs + Vitamin D. Stool samples were collected before, during, and after treatment. The outcomes measured were microbiome colonization, immune profile changes, safety, and tolerability.
Duration
4 weeks of supplementation.
Data Collection
Stool samples, DNA/RNA tube samples, weekly questionnaires, and complex scheduled at-home journal entries.
Software Product
Chloe Clinical (Saas model) was the sole software vendor.
Why Chloe?
Scheduled Forms
Chloe enabled the automatic delivery of time-sensitive forms, minimizing participant burden and ensuring adherence to protocol. Using day-of-week scheduling (Mon, Wed, Fri) made study diaries predictable and manageable for working parents. This represented a significant improvement compared to the practice of sending forms a set number of days after enrollment.
Data Analysis Support
Chloe’s automated generation of occurrence counts allowed Persephone to repeat certain forms while providing a clear record of how many items had been asked of participants. This eliminated manual tracking and streamlined the data analysis stage.
Passive Data Collection
Participants could input their data and log adverse events or symptom changes at any time.
Compliance Tracking
Built-in compliance monitoring tools allowed study coordinators to view participant activity, identify engagement patterns, and follow up on missed entries—ensuring high data integrity and adherence throughout the trial.
Chloe vs. Competitors
User Experience
Lab assistant Jasmine Tinoco shared that when it came to “completing forms in Chloe versus REDCap…your software is a lot better for the user.” Past experiences with other platforms had revealed participant frustrations, technical problems, and high drop-out risks due to confusing interfaces.
Mobile Access
The ability to allow participants to complete tasks on their phones was a major factor in Chloe's selection.
High Value
Chloe was recognized for delivering excellent value at a relatively affordable cost.
Study Design Flexibility
Chloe supported all of the nuanced protocol design features needed to run ARTEMIS.
Persephone’s Process
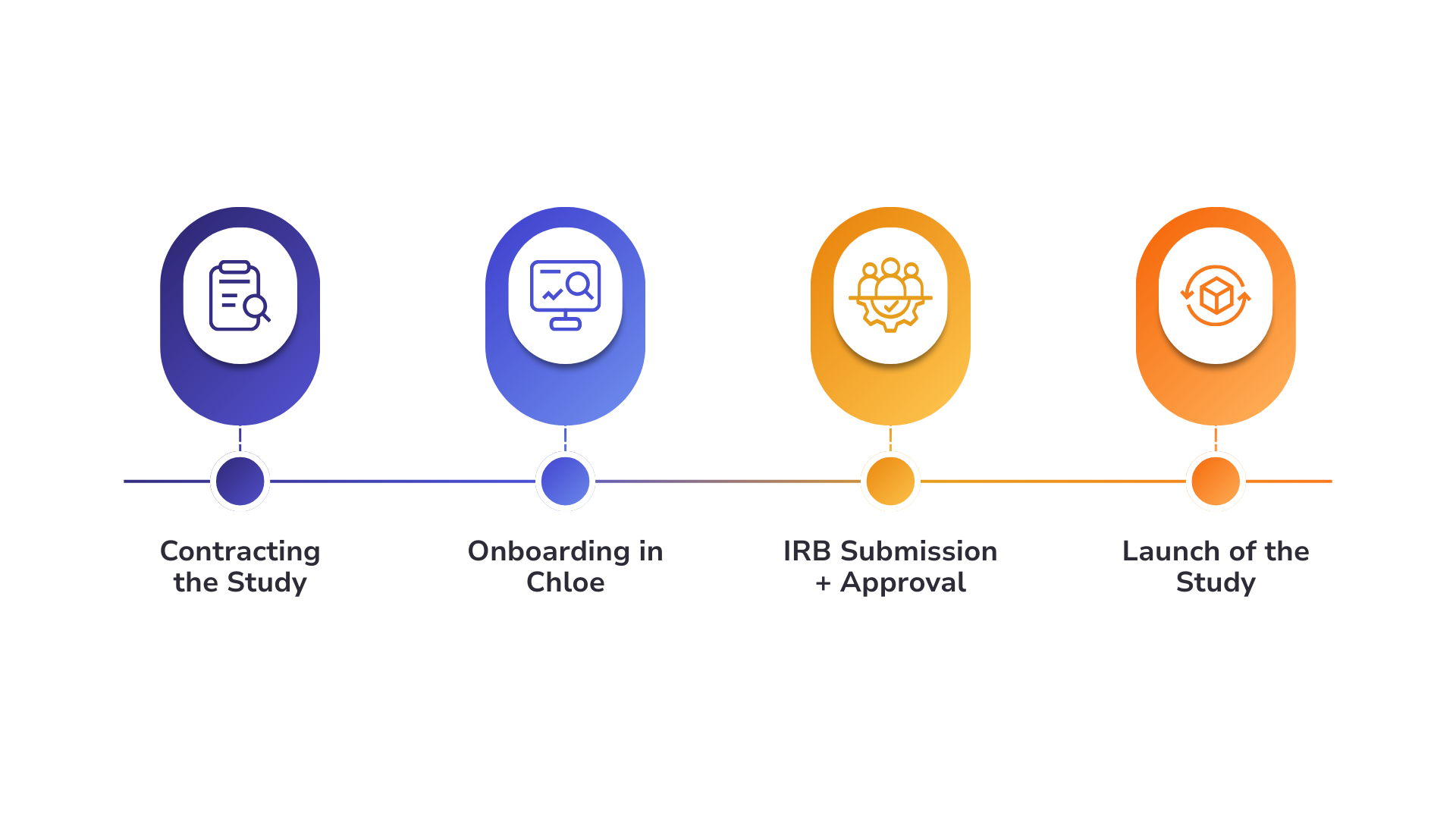
The ARTEMIS study was contracted in August of 2024.
The Persephone team onboarded in September and learned how to build out Chloe themselves, which included designing all of their forms. The team reported a smooth onboarding experience, sharing that they received a clear overview of how to use the platform.
The team independently secured IRB approval in preparation for study execution.
ARTEMIS was launched in January of 2025.
When evaluating EDC platforms, our primary criteria were ease of use for the participants and ability to access on a mobile app. Other factors influencing our decision were price (of course!), ease of configuring the participant journey and entering our questionnaires, flexibility in questionnaire format, and availability of support. Chloe ranked high in all these areas, so we went forward with it!
Dr. Stephen Van Dien, PhD
Co-founder and CTO at Persephone Biosciences
Benefits to Persephone
-
By running the ARTEMIS infant study with Chloe, Persephone demonstrated the ability to independently manage a full clinical trial—recruitment, compliance, and data management—without relying on a CRO. Partnering with People Science gave them a faster, more cost‑effective path to high‑quality infant data. Furthermore, the streamlined process ensured strong family engagement and retention in this sensitive population.
-
Secure, HIPAA-compliant infrastructure for rigorous, publication-ready research.
-
Visibility on participant compliance allowed for timely adjustments to maximize study effectiveness.
-
Improved participant experience, boosting retention during the trial.
-
Chloe was incorporated into Persephone’s existing tools (e.g. Typeform, Salesforce, Google Sheets) for custom workflows.
Benefits to
Trial Participants
-
Chloe minimized participant burden with user-friendly interfaces and streamlined data collection. The fact that participants were able to complete forms at home or on the go was critical in maintaining compliance.
-
Real-time feedback on individual progress, making the study experience more engaging.
-
ARTEMIS participants became part of cutting-edge health research. This experience enabled both infants and parents to learn about their health and contribute to scientific advancement without added burden.
Chloe met all of our expectations—which is saying a lot, because no other provider has!
Persephone Biosciences Team
About Persephone Biosciences
Persephone Biosciences is a pioneer in the synbiotic space dedicated to reimagining patient and infant health. They focus on restoring and supporting the complex gut microbiome, which is critical for immunity, digestion, and development. Recent research shows that many infants today lack this resilient gut microbiome rich in protective bacteria, putting them at greater risk for food sensitivities, eczema, asthma, allergies, weakened immunity, and chronic inflammation. Through the ARTEMIS study—a rigorously designed, double-blind, placebo-controlled trial—Persephone is investigating a synbiotic supplement (combining probiotics and prebiotics) aimed at promoting long-term infant gut health and immunity. This innovative research embodies Persephone’s commitment to solutions that support foundational health from day one.
Other Case Studies

Book a Demo
Turn your customers’ everyday health data into your most powerful competitive asset. Explore our Chloe platform today.