CASE STUDY
Efficient Innovation:
People Science and Verb Biotics Evaluate Impact of Novel GABA Probiotic on Sleep Quality
Study Overview
A look at the study facts, key findings, and real-world implications.
Study Snapshot
Primary Focus
Evaluate the impact of 5 Billion CFU daily of Lp815® on subjective and objective measures of sleep in a population with sleep disturbance.
Participant Criteria
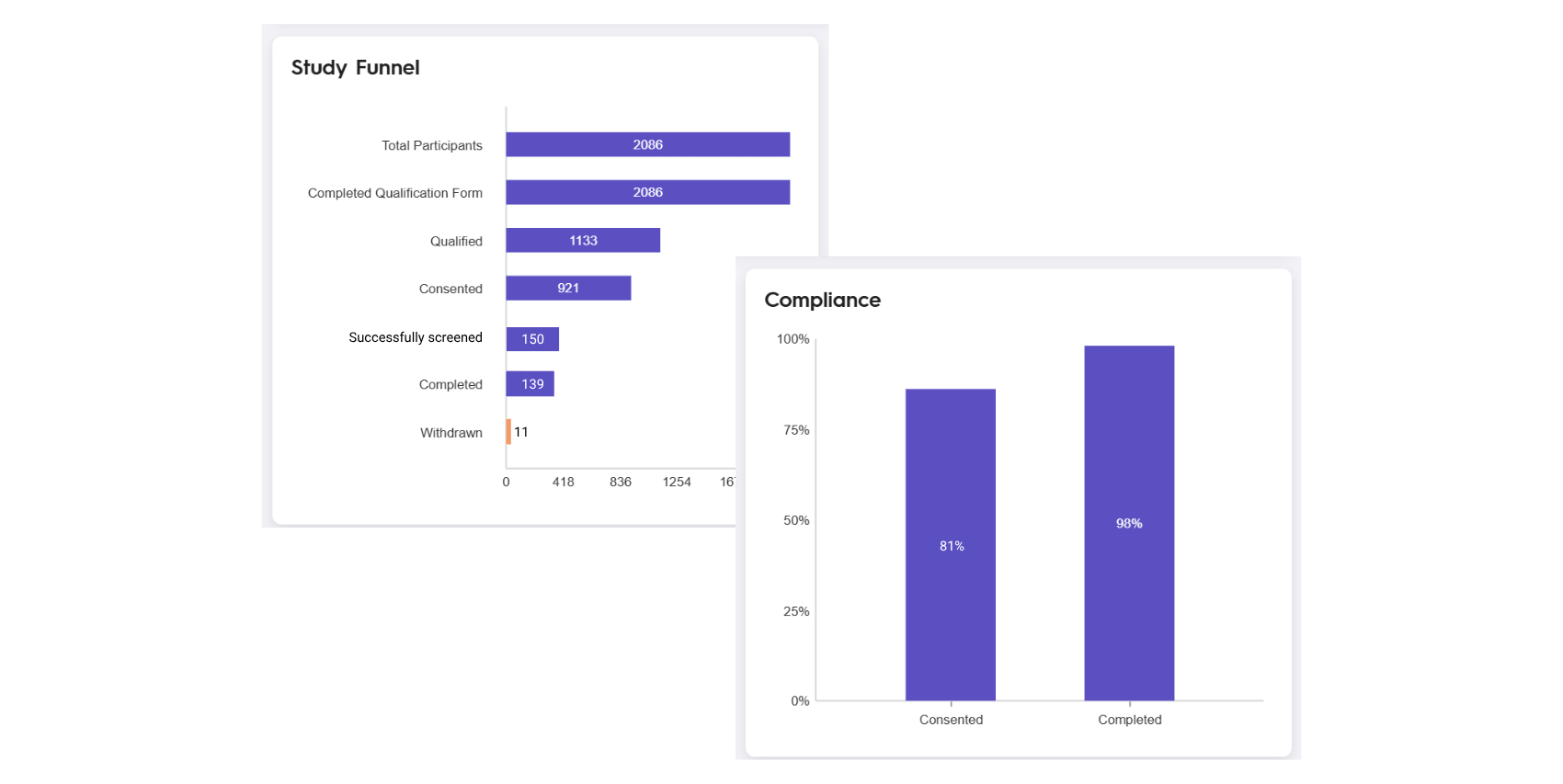
Adults with moderate to severe sleep disruption who met specific eligibility requirements including an Insomnia Severity Index score of > 15, stable medication use, and washout periods for sleep aids and gut health supplements. 150 participants were recruited in 2 weeks, of which 139 were evaluated and 11 excluded.
Study Type
Randomized, placebo-controlled, double-blinded, decentralized trial approved by the Sterling Institutional Review Board (IRB).
Study Structure
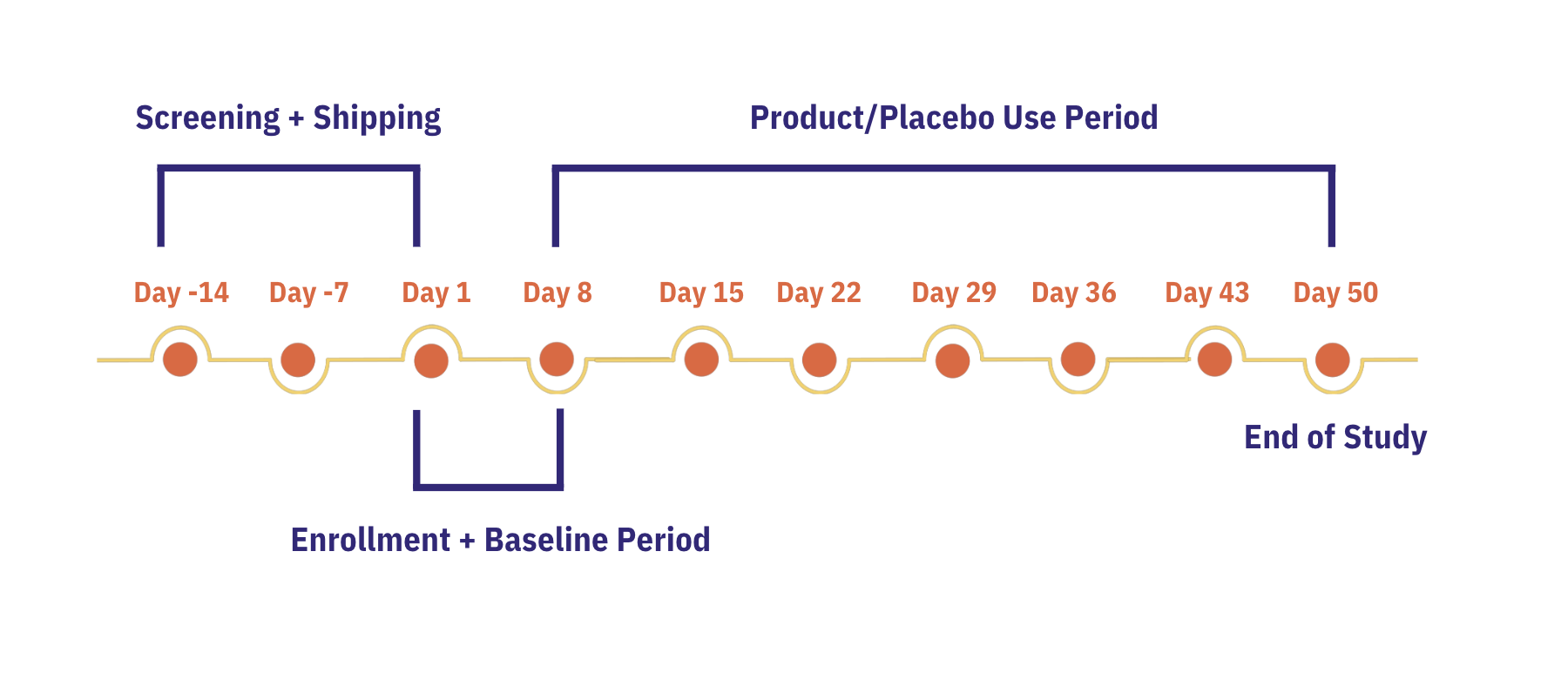
Participants collected 1 week of baseline data followed by 6 weeks of product or placebo use. The study was completely decentralized, with all outcomes logged via the Chloe and Oura Ring mobile apps.
Duration
9-week study; 6-week trial period.
Analytical Methods
Normality was assessed using the Shapiro-Wilk (SW) test. Mann-Whitney U tests (BH-FDR correction) and GLMMs were used on non-normally distributed data. Mann-Kendall trend over time used in daily data.
Validation
A manuscript describing the findings is published at Nature Scientific Reports and available here, DOI: 10.1038/s41598-025-27861-6 .
Key Results
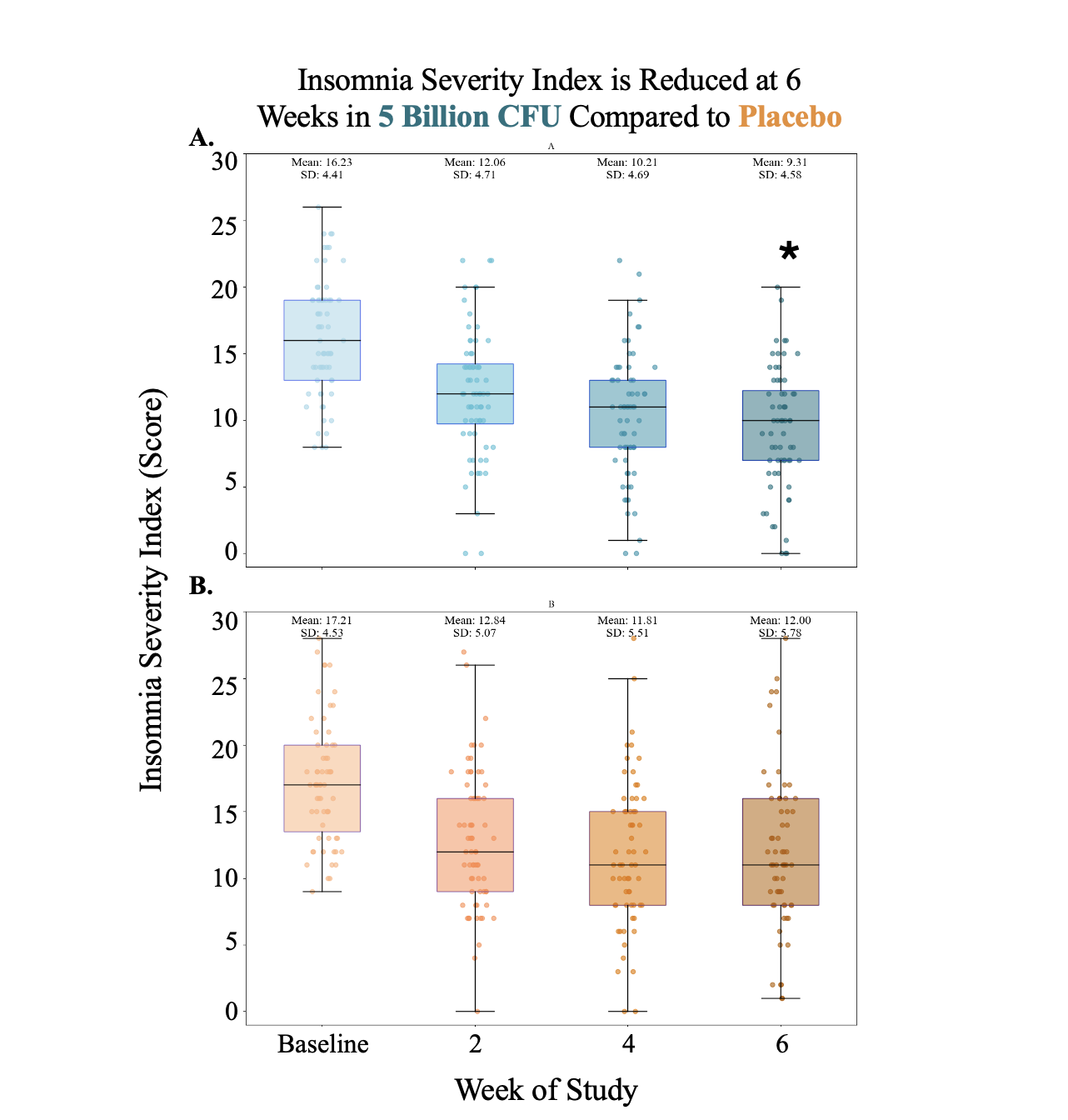
77.3%
Participants Improved their Insomnia Severity Index Scores by 4+ Points
p < 0.05
Reduced Intermittent Anxiety at 2, 4, and 6 Weeks with Greater Benefits for Women
Sleep
Improved Night Sweats and Reduced Difficulty Falling and Staying Asleep
GABA
Significantly Increased Urinary GABA After 7 Days of Intake
89%
Participants Would Participate in Another People Science Study
Study Details
Introduction
Gamma-aminobutyric acid (GABA) is the body’s primary inhibitory neurotransmitter, integral to both mood and sleep regulation. It is produced both in the brain and in the periphery, including by gut microbiota. In a previous study, Verb Biotics — a leader in next-generation probiotic development — partnered with People Science to investigate whether lactiplantibacillus plantarum Lp815® improves symptoms of anxiety. Lp815® is a strain selected in the laboratory to produce the inhibitory neurotransmitter GABA in the physiologic pH range. The results marked a breakthrough, as Lp815® intake was associated with a significant reduction in symptoms of intermittent anxiety compared to placebo.
Building on those findings, Verb Biotics and People Science partnered to evaluate Lp815®’s impact on subjective and wearable sleep data, mood, and digestion over 7 weeks in adults with self-reported sleep disturbance. The project included a sub-study on urinary GABA excretion to tie subjective and objective sleep and mood outcomes to the proposed mechanism of action: increased peripheral GABA. Since many current sleep support products have substantial risk profiles, this safe probiotic is a promising alternative. By enhancing gut-derived GABA production and modulating the gut-brain axis, Lp815® could provide a novel approach to addressing sleep and intermittent anxiety disorders.
Execution
To execute a decentralized, double-blind, randomized, placebo-controlled study, People Science utilized its proprietary Consumer Health Learning & Organizing Ecosystem (Chloe). Chloe combines a mobile app with a health research platform designed for companies that prioritize scientific rigor, enabling rapid and cost-effective validation of product claims through evidence-based methods.
Chloe streamlined study design, data collection, study communications and data visualization, providing a comprehensive approach to participatory research.
Recruitment targeted adults with poor sleep symptoms, assessed by the Insomnia Severity Index, and leveraged digital outreach strategies to enroll a diverse population. A total of 139 participants were included (51.1% female, 55.4% Caucasian) in the 9-week study.
Data Collection
Participants took part in in a 9-week study involving screening, randomization, a one-week baseline, and six weeks of daily product/placebo use. A subset of 17 participants also contributed urine samples for neurotransmitter analysis.
During baseline, demographics, medical history, anxiety (GAD-7), Insomnia Severity Index scores (ISI), cognition, and wearable data were logged through the Chloe mobile app. Participants were randomized in a double-blind manner to receive either 5 billion CFU of Lactiplantibacillus plantarum Lp815 or placebo daily. Outcome measures incorporated both subjective reports (sleep, mood, anxiety, digestion) and objective sleep metrics collected through the Gen3 Oura Ring.
Study Outcome
The collaboration yielded statistically significant, publishable results.
Clinical Validation: While ISI scores were similar at baseline and improved in both groups, there was a significantly greater reduction in poor sleep severity in the treatment group by week 6. Additionally, GAD-7 scores (measuring symptoms of anxiety) decreased more in the 5 Billion CFU group at weeks 2, 4, and 6 compared to placebo.
Decentralized Trial Efficiency: Utilizing the Chloe app minimized logistical challenges and improved participant engagement, setting a flexible model for future research.
Effective Execution: Completed the trial over a 9-week period. The project demonstrated People Science’s full-service support capabilities, from study design to data analysis.
“We entered clinical research with a strong foundation in microbiome science and a clearly defined target population. Collaborating with People Science allowed us to apply that expertise within a rigorous, data-driven framework. Their scientific integrity and analytical depth aligned with our commitment to advancing the evidence base for next-generation biotics with measurable impact on human health.”
Noah Zimmerman, PhD
CSO at Verb Biotics
Benefits to Verb Biotics
-
This RCT provided robust evidence that Verb’s GABA probiotic product improves sleep and anxiety—two of the most prevalent and interconnected wellness concerns today.
These findings position Verb’s product as both effective and highly relevant in a market seeking safe, non-pharmaceutical alternatives. By investing in gold-standard clinical research, Verb has established itself as a science-first supplier, improving its standing with both current and prospective partners across the health and wellness industry. Trial results are published in Nature Scientific Reports (DOI: 10.1038/s41598-025-27861-6 ), strengthening Verb’s credibility and garnering media attention for their GABA-producing probiotic.
-
Enabled seamless remote participation, reducing logistical barriers and costs associated with in-person visits.
-
Provided instant insights into participant data, allowing timely adjustments to maximize study effectiveness.
-
Structured prompts and reminders helped maintain high data quality and participant adherence throughout the trial.
-
All participants could view their own results after the study. Putting participant experience at the center of our studies means high participant satisfaction, high compliance, and a growing pool of community participants.
-
Real-time feedback on individual progress, capturing more of participants’ interest.
-
89% of participants said they would participate in another People Science study, and recommend People Science studies to friends and family.
Benefits to
Trial Participants
“This was a very well organized study! Thank you for allowing me to be involved in this research!!”
Study Participant
Other Case Studies

Book a Demo
Turn your customers’ everyday health data into your most powerful competitive asset. Explore our platform Chloe today.